5.0 X 10
interactiveleap
Sep 18, 2025 · 6 min read
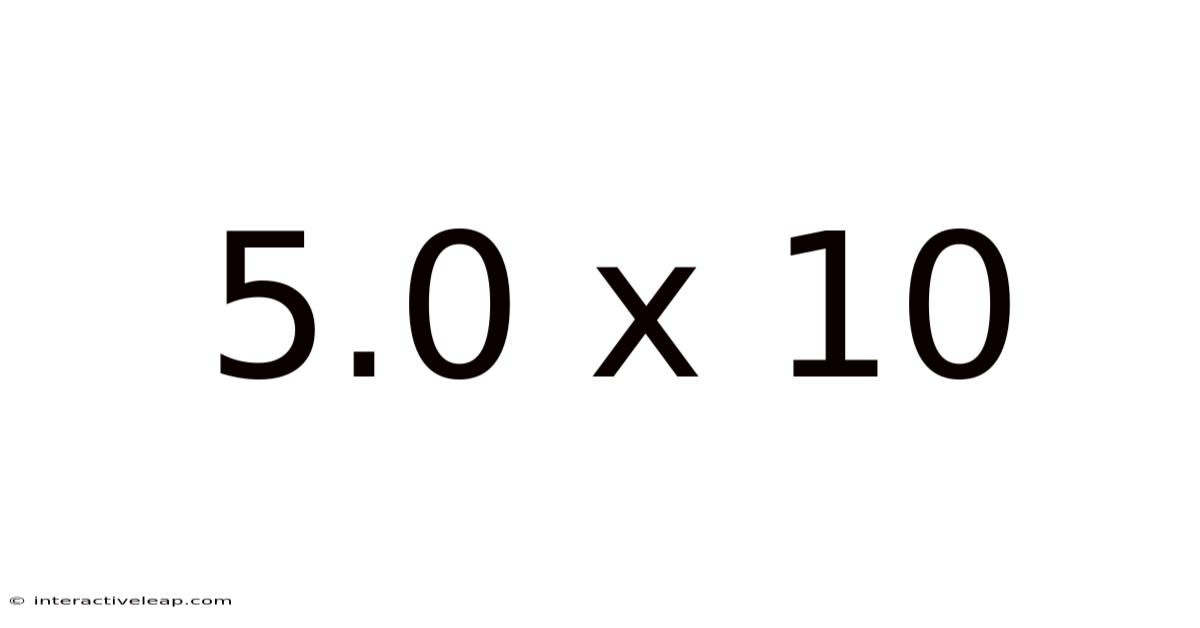
Table of Contents
Decoding 5.0 x 10: Understanding Scientific Notation and its Applications
Scientific notation, a cornerstone of scientific and engineering fields, allows us to represent extremely large or extremely small numbers in a concise and manageable format. Understanding this system is crucial for grasping concepts across various disciplines, from astrophysics dealing with immense distances to nanotechnology working with minuscule particles. This article will delve into the intricacies of scientific notation, focusing specifically on the number 5.0 x 10, exploring its meaning, applications, and broader implications within the framework of scientific representation.
Understanding Scientific Notation
Scientific notation expresses numbers in the form of a coefficient multiplied by a power of 10. The coefficient is always a number between 1 and 10 (but not including 10), and the power of 10 indicates the magnitude of the number. For instance, the number 5,000,000 can be written in scientific notation as 5.0 x 10<sup>6</sup>. Here, 5.0 is the coefficient, and 10<sup>6</sup> signifies that the decimal point should be moved six places to the right.
Conversely, a small number like 0.000005 can be expressed as 5.0 x 10<sup>-6</sup>. The negative exponent indicates that the decimal point should be moved six places to the left. This system elegantly handles numbers that would be cumbersome to write in standard decimal notation.
The Significance of 5.0 x 10
While seemingly simple, the expression 5.0 x 10 carries significant implications depending on the context. The crucial part here is the absence of an exponent. This means we're dealing with a number that doesn't require shifting the decimal place for magnitude adjustment. In essence, 5.0 x 10 is simply 50.
However, the use of scientific notation in this instance suggests a specific purpose. It might be used to maintain consistency in calculations or presentations involving much larger or smaller numbers. For example, in a series of calculations where other numbers are represented in scientific notation, using 5.0 x 10 maintains uniformity and avoids potential errors.
Applications Across Disciplines
The use of scientific notation, even for seemingly straightforward numbers like 50, extends far beyond just mathematical convenience. Let's explore some key applications:
-
Physics: In physics, dealing with quantities like mass, velocity, and acceleration often involves extremely large or small values. Using scientific notation simplifies these calculations and reduces errors. Consider the speed of light, approximately 3.0 x 10<sup>8</sup> m/s. Representing this in standard notation would be cumbersome and prone to mistakes.
-
Chemistry: Chemistry deals with incredibly small particles, like atoms and molecules. The mass of a single atom of hydrogen is approximately 1.67 x 10<sup>-27</sup> kg. Scientific notation allows chemists to work effectively with these minuscule measurements without losing track of significant figures.
-
Astronomy: Astronomy tackles astronomical distances and masses. The distance to the sun, for instance, is roughly 1.5 x 10<sup>11</sup> meters. Using scientific notation prevents these figures from becoming unwieldy and facilitates calculations involving celestial bodies.
-
Computer Science: Computer science deals with large data sets and memory capacity, often expressed in terms of bytes, kilobytes, megabytes, and so on. Scientific notation aids in efficiently representing these vast amounts of data. The capacity of a hard drive, for example, might be expressed as 2.0 x 10<sup>12</sup> bytes.
-
Engineering: Engineering designs often involve scaling, where dimensions need to be multiplied or divided by large factors. Scientific notation ensures precise calculations and minimizes rounding errors, crucial for designing structures and machines that need to function accurately.
Calculations with Scientific Notation
Working with scientific notation requires understanding the rules of exponents. When multiplying numbers in scientific notation, we multiply the coefficients and add the exponents. When dividing, we divide the coefficients and subtract the exponents.
Example:
(2.0 x 10<sup>3</sup>) x (3.0 x 10<sup>2</sup>) = (2.0 x 3.0) x 10<sup>(3+2)</sup> = 6.0 x 10<sup>5</sup>
(6.0 x 10<sup>5</sup>) / (2.0 x 10<sup>3</sup>) = (6.0 / 2.0) x 10<sup>(5-3)</sup> = 3.0 x 10<sup>2</sup>
These rules are fundamental to carrying out complex calculations that involve vastly different scales of magnitude, seamlessly handled using scientific notation.
Significant Figures and Scientific Notation
The number of significant figures in a measurement reflects the precision of the measurement. Scientific notation helps maintain accuracy by clearly indicating significant figures. For example, 5.0 x 10 indicates two significant figures, whereas 5 x 10 only has one. This distinction is vital in scientific and engineering applications, where precision matters immensely.
Converting to and from Scientific Notation
Converting a number to scientific notation involves moving the decimal point until a single non-zero digit remains to the left of it. The number of places the decimal point is moved determines the exponent of 10. If the decimal point is moved to the left, the exponent is positive; if moved to the right, it's negative.
Example:
Converting 5000 to scientific notation: Move the decimal point four places to the left to get 5.0. Therefore, 5000 = 5.0 x 10<sup>4</sup>
Converting 0.0005 to scientific notation: Move the decimal point four places to the right to get 5.0. Therefore, 0.0005 = 5.0 x 10<sup>-4</sup>
Frequently Asked Questions (FAQ)
Q: Why is scientific notation important?
A: Scientific notation provides a concise and manageable way to represent extremely large or small numbers, which are common in many scientific and engineering fields. It also simplifies calculations and reduces errors.
Q: How do I add or subtract numbers in scientific notation?
A: Before adding or subtracting, you need to express the numbers with the same exponent of 10. Then, add or subtract the coefficients and keep the exponent the same.
Q: What are significant figures and why are they important in scientific notation?
A: Significant figures represent the precision of a measurement. Scientific notation clearly displays significant figures, ensuring accuracy in calculations and reporting results.
Q: Can I use a calculator to work with scientific notation?
A: Most scientific calculators have a built-in function to handle scientific notation, making calculations easier and more efficient.
Q: What if the coefficient is not between 1 and 10?
A: If the coefficient is not between 1 and 10, adjust the exponent accordingly to bring the coefficient into the correct range.
Conclusion
The seemingly simple expression 5.0 x 10, though representing the number 50, highlights the power and utility of scientific notation. Its application extends far beyond simple numerical representation. It is an essential tool for handling vast ranges of numerical values encountered across diverse scientific and engineering domains. Understanding scientific notation and its underlying principles is fundamental for anyone pursuing studies or careers in STEM fields. By mastering this system, one gains the ability to work efficiently and accurately with numbers spanning vast magnitudes, contributing to a more robust understanding and application of scientific concepts. From the infinitesimally small to the astronomically large, scientific notation serves as a universal language for quantifying the universe around us.
Latest Posts
Latest Posts
-
4x5 In Pixels
Sep 18, 2025
-
25 Of 8
Sep 18, 2025
-
75 Of 36
Sep 18, 2025
-
3000g To Lbs
Sep 18, 2025
-
Low Cost Wigs
Sep 18, 2025
Related Post
Thank you for visiting our website which covers about 5.0 X 10 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.