Fe Oh 3
interactiveleap
Sep 23, 2025 · 7 min read
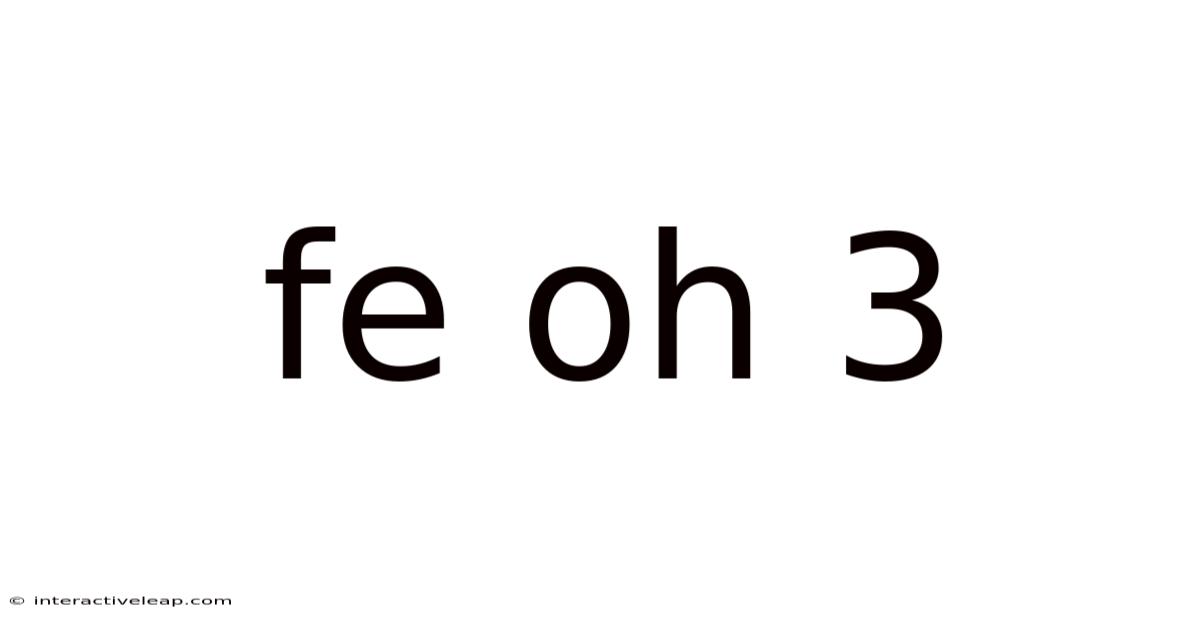
Table of Contents
Unveiling the Mysteries of Fe(OH)₃: Properties, Synthesis, and Applications
Iron(III) hydroxide, often represented as Fe(OH)₃, is a fascinating compound with a wide range of applications, from water purification to pigments. Understanding its properties, synthesis methods, and diverse uses is crucial for various scientific and industrial fields. This comprehensive guide delves into the world of Fe(OH)₃, exploring its intricate chemical behavior and significant roles in different applications.
Introduction to Iron(III) Hydroxide, Fe(OH)₃
Fe(OH)₃, commonly known as ferric hydroxide, is an inorganic compound characterized by its reddish-brown color and gelatinous nature. It's not a simple, well-defined crystalline solid like many other metal hydroxides but rather exists as a hydrated oxide, often represented as FeO(OH)·xH₂O, where 'x' denotes a variable amount of water molecules. This inherent variability makes its precise characterization challenging, contributing to the diverse forms it can adopt. This article explores the different facets of Fe(OH)₃, covering its synthesis, properties, and most importantly, its wide-ranging applications. Understanding Fe(OH)₃ is essential for anyone working in fields like chemistry, materials science, and environmental engineering.
Properties of Fe(OH)₃
Fe(OH)₃ boasts a unique set of properties that contribute to its diverse applications. These properties are intricately linked to its structure and chemical behavior.
Physical Properties:
- Appearance: Typically a reddish-brown, gelatinous precipitate.
- Solubility: Insoluble in water, but soluble in acids and strong bases. This amphoteric nature is a defining characteristic.
- Density: Varies depending on hydration and crystalline structure.
- Melting Point: Decomposes upon heating rather than melting, converting to iron(III) oxide (Fe₂O₃).
Chemical Properties:
- Amphoteric Nature: Fe(OH)₃ reacts with both acids and bases, showcasing its amphoteric character. It dissolves in acids to form iron(III) salts, and in strong bases to form ferrate(III) ions ([Fe(OH)₆]³⁻). This dual reactivity is critical in various chemical processes.
- Dehydration: Upon heating, Fe(OH)₃ readily loses water molecules, transforming into iron(III) oxide (Fe₂O₃). This dehydration reaction is crucial in the production of iron oxide pigments.
- Oxidation-Reduction Reactions: Fe(III) can undergo reduction to Fe(II) under specific conditions, affecting its reactivity in redox reactions.
- Coordination Chemistry: Fe(III) is a transition metal ion, capable of forming coordination complexes with various ligands, significantly influencing its chemical behavior and reactivity.
Synthesis of Fe(OH)₃
The synthesis of Fe(OH)₃ is relatively straightforward, typically involving the precipitation reaction between a soluble iron(III) salt and a base. Various methods exist, each offering advantages and disadvantages.
Common Synthesis Methods:
-
Precipitation from Aqueous Solution: This is the most common method, involving the addition of a strong base (such as sodium hydroxide, NaOH, or ammonia, NH₃) to an aqueous solution containing a soluble iron(III) salt (like iron(III) chloride, FeCl₃, or iron(III) nitrate, Fe(NO₃)₃). The reaction can be represented as:
Fe³⁺(aq) + 3OH⁻(aq) → Fe(OH)₃(s)
The resulting precipitate is typically washed and dried to obtain the desired product. The purity and properties of the final product heavily depend on reaction conditions, such as temperature, pH, and concentration of reactants.
-
Hydrothermal Synthesis: This method employs high temperatures and pressures to control the crystal growth and morphology of Fe(OH)₃. It allows for the synthesis of highly crystalline and well-defined particles with specific properties tailored for specific applications.
-
Sol-Gel Method: This technique involves the formation of a colloidal solution (sol) containing iron(III) precursors, followed by gelation and subsequent heat treatment to obtain the desired Fe(OH)₃ material. This method is often used to synthesize Fe(OH)₃ nanoparticles with controlled size and shape.
Applications of Fe(OH)₃
The diverse properties of Fe(OH)₃ make it a valuable compound with applications across numerous fields.
1. Water Treatment:
Fe(OH)₃ plays a crucial role in water purification as a coagulant and flocculant. Its ability to adsorb impurities and form flocs allows for the efficient removal of suspended solids, colloids, and other contaminants. The addition of Fe(OH)₃ to water causes small particles to clump together, forming larger flocs that can be easily separated by sedimentation or filtration. This is a cornerstone of many municipal water treatment processes.
2. Pigment Production:
The reddish-brown color of Fe(OH)₃, and its derivative iron(III) oxide (Fe₂O₃), makes it an important pigment in various applications. After dehydration, it finds use in paints, coatings, and cosmetics, offering a cost-effective and durable coloring agent.
3. Catalysis:
Fe(OH)₃ and its derivatives exhibit catalytic activity in several chemical reactions. Its ability to facilitate chemical transformations makes it a valuable component in various catalytic processes. This property is being explored in areas like oxidation reactions and organic synthesis.
4. Battery Materials:
Research is exploring the potential of Fe(OH)₃ in battery technology, specifically in developing high-capacity and cost-effective electrodes for rechargeable batteries. Its redox properties make it a promising material for energy storage applications.
5. Environmental Remediation:
Fe(OH)₃ shows promise in environmental remediation due to its ability to adsorb various pollutants from contaminated water and soil. Its use in removing heavy metals and other harmful substances is being actively investigated.
6. Medicine:
While less prevalent compared to other applications, Fe(OH)₃ has shown some potential in medicinal applications, primarily related to its ability to bind to certain toxins and facilitate their removal from the body. However, this area requires further research and development.
Safety Precautions When Handling Fe(OH)₃
While Fe(OH)₃ itself is generally considered non-toxic, certain precautions should be taken when handling it, especially in powdered form:
- Avoid inhalation: Inhalation of fine Fe(OH)₃ powder can irritate the respiratory system. Appropriate respiratory protection should be used.
- Eye protection: Wear safety glasses to prevent eye irritation from contact with Fe(OH)₃.
- Skin contact: Avoid prolonged skin contact, as it may cause mild irritation. Wash thoroughly with soap and water after handling.
- Disposal: Dispose of Fe(OH)₃ waste according to local regulations to prevent environmental contamination.
Frequently Asked Questions (FAQ)
Q1: What is the difference between Fe(OH)₃ and Fe₂O₃?
A1: Fe(OH)₃ is iron(III) hydroxide, a hydrated form containing water molecules. Upon heating or dehydration, it converts to Fe₂O₃, iron(III) oxide, which is an anhydrous form lacking water molecules. Both are iron(III) compounds but differ in their water content and properties.
Q2: Is Fe(OH)₃ a strong or weak base?
A2: Fe(OH)₃ is not a strong base; rather it displays amphoteric behavior, reacting with both acids and bases. Its solubility in acids and strong bases demonstrates this characteristic.
Q3: What are the common impurities found in synthesized Fe(OH)₃?
A3: The purity of synthesized Fe(OH)₃ depends heavily on the synthesis method and the purity of starting materials. Common impurities can include other iron oxides, hydroxides of other metals present as contaminants in the starting materials, and residual salts from the reaction.
Q4: How is the size and morphology of Fe(OH)₃ particles controlled during synthesis?
A4: The size and morphology of Fe(OH)₃ particles can be controlled by adjusting various parameters during synthesis, including reaction temperature, pH, concentration of reactants, and the presence of additives (surfactants or templates). Hydrothermal synthesis and sol-gel methods allow for greater control over these parameters.
Q5: What are the future prospects of Fe(OH)₃ research?
A5: Future research on Fe(OH)₃ will likely focus on further optimizing its use in water treatment, developing its applications in advanced battery technologies, and exploring its potential in catalysis and environmental remediation. Understanding its structure-property relationships at the nanoscale will also be crucial for tailoring its properties for specific applications.
Conclusion
Iron(III) hydroxide, Fe(OH)₃, is a versatile compound with a wide range of applications stemming from its unique physical and chemical properties. Its amphoteric nature, ability to act as a coagulant, and potential in various catalytic and environmental remediation processes makes it a crucial material in various industries. While its synthesis is relatively simple, understanding the nuances of its preparation and handling is critical for maximizing its utility and ensuring safety. As research continues, the importance of Fe(OH)₃ in diverse technological applications is only set to grow. Further investigations into its nanostructured forms and advanced synthesis techniques will undoubtedly unlock even more exciting possibilities for this fascinating compound.
Latest Posts
Latest Posts
-
Lutheran Vs Catholicism
Sep 23, 2025
-
Layout Roman Villa
Sep 23, 2025
-
45 Of 800
Sep 23, 2025
-
Tap And Wrench
Sep 23, 2025
-
173lbs To Kg
Sep 23, 2025
Related Post
Thank you for visiting our website which covers about Fe Oh 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.