Cr Oh 3
interactiveleap
Sep 22, 2025 · 7 min read
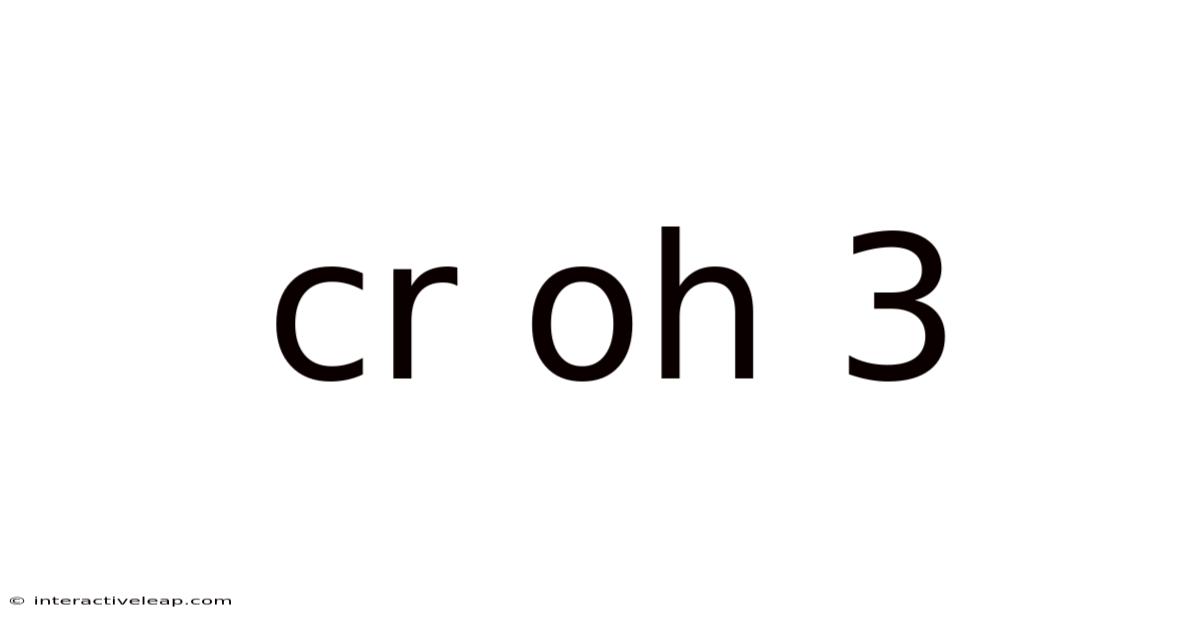
Table of Contents
Delving Deep into Chromium(III) Oxide (Cr₂O₃): Properties, Applications, and Safety
Chromium(III) oxide, also known as chromic oxide, is a dark green, inorganic compound with the chemical formula Cr₂O₃. This fascinating material possesses a unique blend of properties that make it invaluable in a wide array of industrial applications, from pigments and catalysts to polishing compounds and refractory materials. Understanding its characteristics, production methods, and safety considerations is crucial for anyone working with or studying this important chemical. This comprehensive article will explore all these facets of Cr₂O₃, providing a detailed and insightful look at this versatile compound.
Introduction to Chromium(III) Oxide (Cr₂O₃)
Chromium(III) oxide exists in several crystalline forms, the most common being the α-form, which possesses a corundum-like structure. This structure contributes significantly to its high hardness, chemical inertness, and thermal stability. These properties are instrumental in its various uses. It's crucial to differentiate Cr₂O₃ from other chromium compounds, particularly chromium(VI) oxide (CrO₃), which is highly toxic. Cr₂O₃, while not entirely innocuous, is considerably less hazardous. This difference highlights the importance of accurate identification and handling procedures when working with chromium compounds. The key applications of Cr₂O₃ stem directly from its unique characteristics, making it a cornerstone material in diverse industries.
Key Properties of Chromium(III) Oxide
Several key properties make Cr₂O₃ a desirable material in numerous applications:
-
Color: Its intense, vibrant green color is perhaps its most recognizable characteristic. This makes it a prized pigment in paints, plastics, and ceramics.
-
Chemical Inertness: Cr₂O₃ exhibits excellent resistance to chemical attack, making it suitable for use in harsh environments. This property is vital in its application as a catalyst and in high-temperature applications.
-
Thermal Stability: It possesses high thermal stability, meaning it can withstand high temperatures without significant degradation. This makes it a valuable component in refractory materials and high-temperature applications.
-
Hardness: Its hardness, similar to corundum (Al₂O₃), makes it an effective abrasive in polishing compounds.
-
Magnetic Properties: Cr₂O₃ exhibits weak antiferromagnetic properties at room temperature, but becomes paramagnetic at temperatures above its Néel temperature.
-
Insulating Properties: It's a good electrical insulator, further contributing to its versatility in various applications.
-
Crystalline Structure: The α-form's corundum structure is responsible for many of its desirable mechanical and chemical properties. Other less common crystalline forms exist, but their properties differ and their applications are less widespread.
Production Methods of Chromium(III) Oxide
Several methods are employed for the industrial production of Cr₂O₃:
-
Reduction of Chromium(VI) Oxide: This is a common method, involving the reduction of chromium(VI) oxide (CrO₃) using various reducing agents, such as sulfur, carbon, or hydrogen. This process requires careful control to avoid the formation of other chromium compounds.
-
Thermal Decomposition of Ammonium Dichromate: Heating ammonium dichromate ((NH₄)₂Cr₂O₇) results in its decomposition to Cr₂O₃, nitrogen gas, and water. This method is often used for laboratory-scale synthesis.
-
Oxidation of Chromium Metal: Chromium metal can be oxidized to Cr₂O₃ through careful heating in air or oxygen. This method is less common due to its higher energy requirements.
-
Precipitation from Chromium Salts: Chromium(III) salts, such as chromium(III) chloride (CrCl₃), can be precipitated as hydroxide and then calcined at high temperature to yield Cr₂O₃. Careful control of the precipitation process is vital to ensure high purity.
Applications of Chromium(III) Oxide
The wide range of Cr₂O₃ applications stems directly from its unique properties. Here are some key applications:
-
Pigments: The vibrant green color of Cr₂O₃ makes it a valuable pigment in paints, coatings, plastics, inks, and cosmetics. Its chemical inertness and lightfastness ensure its color remains stable over time.
-
Catalysts: Cr₂O₃ acts as a catalyst in various chemical reactions, notably in the production of synthetic fibers and in oxidation processes. Its catalytic activity is influenced by its surface area and the presence of other elements.
-
Polishing Compounds: Its hardness makes it an excellent abrasive in polishing compounds for metals, glass, and ceramics. This application is particularly common in the metal finishing industry.
-
Refractory Materials: Cr₂O₃'s high melting point and chemical resistance make it a valuable component in refractory materials, which are materials that can withstand high temperatures without significant degradation. These materials are used in furnace linings and other high-temperature applications.
-
Magnetic Recording Media: Cr₂O₃ has historically been used in magnetic recording media, although it's less common now due to the advent of more advanced materials.
-
Ceramics: Cr₂O₃ is added to ceramics to enhance their properties, such as strength, hardness, and color.
Safety Considerations and Environmental Impact
While less toxic than chromium(VI) compounds, Cr₂O₃ still requires careful handling. Inhalation of Cr₂O₃ dust can cause respiratory irritation, and prolonged exposure may lead to more serious health problems. Appropriate safety measures, including the use of respiratory protection and proper ventilation, are necessary when handling Cr₂O₃. Disposal of Cr₂O₃ waste should follow local regulations to minimize environmental impact. The production and use of Cr₂O₃ should strive for sustainability, reducing waste and promoting environmentally friendly practices.
Explanation of the Chemical Properties
The chemical properties of Cr₂O₃ are largely dictated by its electronic structure and crystalline form. The chromium(III) ion (Cr³⁺) possesses a partially filled d-orbital, which contributes to its ability to participate in redox reactions, albeit less readily than chromium(VI) compounds. The strong Cr-O bonds in the corundum structure contribute to its high thermal stability and chemical inertness. Its amphoteric nature means it can react with both acids and bases, although it is relatively unreactive under normal conditions. Dissolution typically requires strong acids and elevated temperatures. The reactivity is heavily dependent on the particle size and surface area of the Cr₂O₃. Finely divided Cr₂O₃ will exhibit higher reactivity than coarser particles.
Frequently Asked Questions (FAQ)
Q: What is the difference between chromium(III) oxide and chromium(VI) oxide?
A: Chromium(III) oxide (Cr₂O₃) is relatively inert and less toxic, while chromium(VI) oxide (CrO₃) is highly toxic and a strong oxidizing agent. The oxidation state of chromium significantly influences its properties and toxicity.
Q: Is chromium(III) oxide carcinogenic?
A: While Cr₂O₃ is less toxic than Cr(VI) compounds, some studies suggest a potential for carcinogenicity, particularly with prolonged exposure to high concentrations of dust. However, the carcinogenic risk is significantly lower than that of Cr(VI) compounds.
Q: What are the environmental concerns associated with chromium(III) oxide?
A: Although less toxic than Cr(VI), improper disposal of Cr₂O₃ can still pose environmental risks. It's important to follow appropriate disposal protocols to prevent contamination of soil and water. The use of Cr₂O₃ should be optimized to minimize waste generation.
Q: How is chromium(III) oxide synthesized in a laboratory setting?
A: Common laboratory methods include the thermal decomposition of ammonium dichromate or the reduction of chromium(VI) oxide using a suitable reducing agent. These methods require careful control of temperature and reaction conditions to obtain high-purity Cr₂O₃.
Q: What are the typical applications of chromium(III) oxide in the ceramics industry?
A: In ceramics, Cr₂O₃ is used as a coloring agent (providing a green hue), a strengthening additive, and to enhance hardness and other desirable mechanical properties. The exact role depends on the specific ceramic formulation.
Conclusion
Chromium(III) oxide is a versatile and valuable inorganic compound with a wide range of applications across diverse industries. Its unique combination of properties – vibrant green color, chemical inertness, thermal stability, and hardness – makes it a cornerstone material in pigments, catalysts, polishing compounds, and refractory materials. While generally less toxic than chromium(VI) compounds, appropriate safety precautions and environmentally responsible handling practices are crucial. Further research into the synthesis of Cr₂O₃ using more sustainable methods and a deeper understanding of its long-term environmental impact will be essential for its continued safe and responsible use. The multifaceted nature of Cr₂O₃ ensures its ongoing relevance in various fields, driving continued innovation and exploration of its potential.
Latest Posts
Latest Posts
-
Heavy Duty Screwdriver
Sep 22, 2025
-
150grams To Oz
Sep 22, 2025
-
Water Soluble Oils
Sep 22, 2025
-
Electrolysis Half Equations
Sep 22, 2025
-
Column Method Subtraction
Sep 22, 2025
Related Post
Thank you for visiting our website which covers about Cr Oh 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.